What is the molecular mass of Na2-sulfate? The answer is not as simple as it sounds. This chemical compound is highly soluble in water and is found in nature in many forms. It has an inorganic formula, Na2SO4, and several related hydrates. The most common form of sodium sulfate is sodium sulfate decahydrate, which is produced annually in the US and is an important commodity chemical product.
To answer the question “What is the molecular mass of Na2So4”, one must first look up the atomic masses of the different elements. Since sodium is a neutral atom and sulphate a negatively charged ion, it takes two sodium atoms to neutralise the negative charge of the sulphate ion. In the next step, we’ll look at the molecular mass of sodium sulphate.

To find the molar mass of Na2SO4, you can use a molar mass calculator. This calculator will give you the common compound name, the atomic weights, and the mass percent. You can also enter the molar mass in grams. You can then use a molar mass calculator to convert the weight of a substance into the number of moles.
Another way to calculate the molecular mass of Na2SO4, Sodium Sulfate, is to use a molar mass calculator. The formula for the molecule can be obtained from the periodic table or a chemical formula. To calculate the molecular mass of a compound, you simply divide its atomic weight by its total mass. Once you have that, you can multiply the molar mass by the atomic weight of each atom.
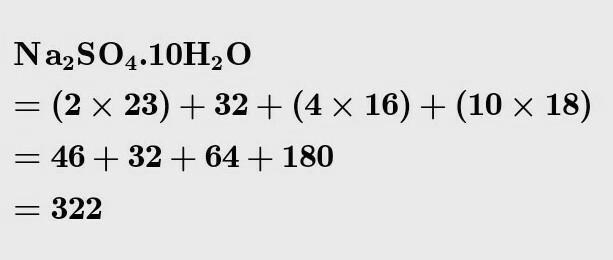
The molecular mass of a chemical substance is also known as the molar mass or molar weight. It is defined as the mass of one mole. The molar mass unit is kilograms per mole, and chemists usually express it in grams per mole. The atomic mass of an element is a single proton or a neutron. A compound’s molar mass is its sum of atoms and molecules.
What is the mass of 10 mol of Na2SO4?
Sodium sulfate (Na2SO4) is a white crystalline solid that is very soluble in water. It has a molecular weight of 142.04 g/mol and is used in a variety of industries, including as a food additive.
To calculate the mass of 10 mol of Na2SO4, we need to multiply the molecular weight by the number of moles.
Molar mass of Na2SO4 = 142.04 g/mol
Number of moles = 10 mol
Therefore, the mass of 10 mol of Na2SO4 is 1,420.4 grams.
What is the molar mass of Na2SO4 apex?
What is the molecular mass of na2so3?
Sodium sulfite (Na2SO3) is the inorganic compound with the molecular formula Na2SO3. It is a white crystalline solid that is soluble in water. It is a salt of sodium sulfite and sulfuric acid.
Sodium sulfite is used in a variety of applications, including as a food preservative, in paper manufacturing, and in the treatment of wastewater.
The molecular mass of Na2SO3 is 126.04 g/mol.
How do I calculate molar mass?
What is formula unit mass?
What is the formula of na2so3?
What is mass of 10 mole of na2so3?
Conclusion
We hope this blog post “What is the Molecular Mass of Na2SO4?” has helped clear up any confusion you may have had. If you have any further questions, feel free to reach out to us and we would be happy to help!
Hey check out: How to Solve the Equation “If 13 x 12 = 651”
Today sponsors are Lifeafter20 and Sprinkler Repair Long Island
Also check out Infinity Charm